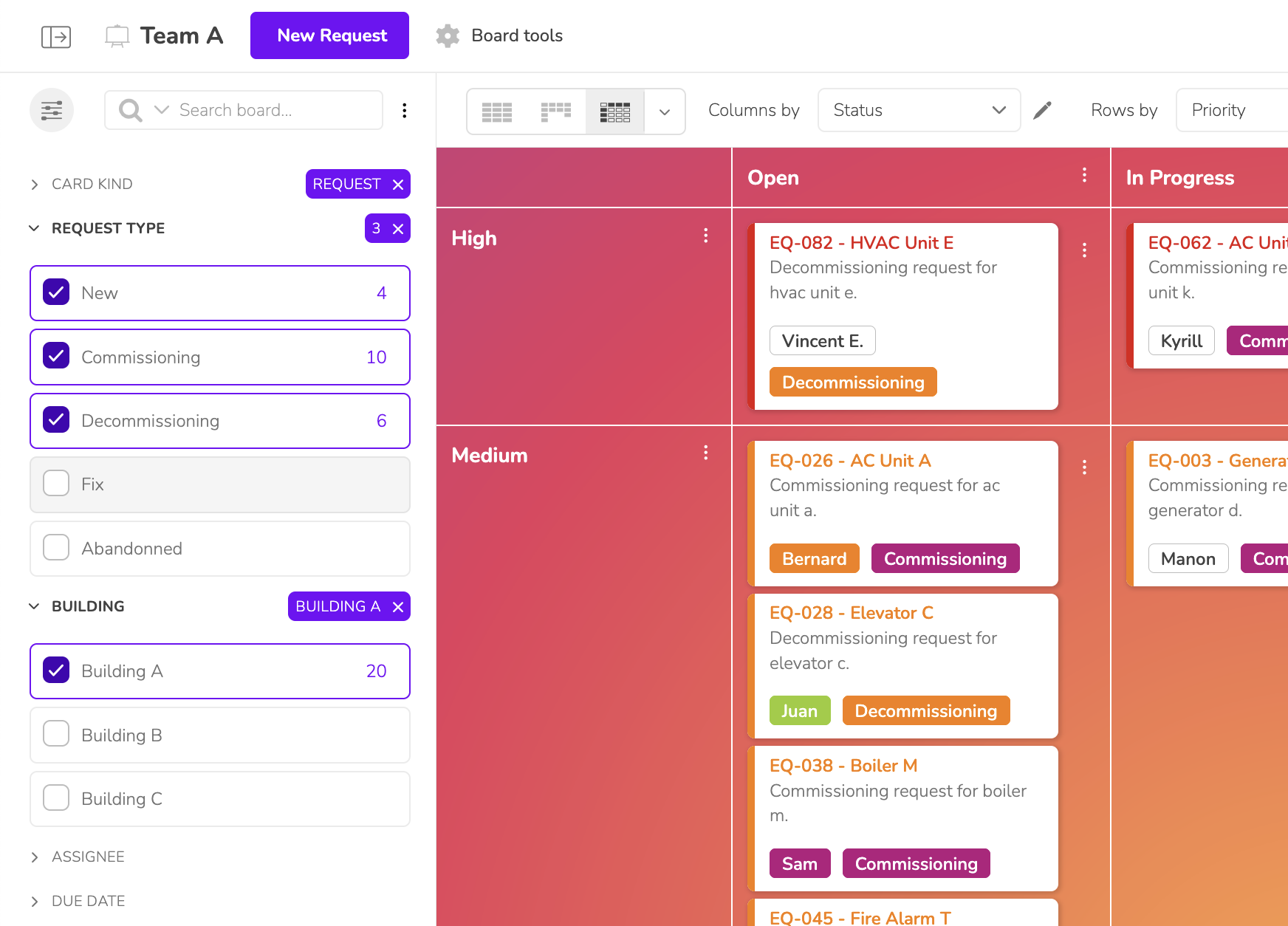
Without a suitable tool, tracking validations and quality controls becomes a nightmare. Every delay can block a batch, complicate an audit, or lead to penalties.
One crashed Excel file, and everything unravels. Lost data, mixed-up versions, scattered information… A single mistake can cause delays of several days.
Teams spend more time searching for files, checking data, or re-entering information than focusing on their mission. The result: declining productivity.
Without centralized tracking, it becomes impossible to ensure full traceability of validations. One audit deviation leads to non-compliance, blocked timelines, and potential sanctions.
Widely used, Excel does not meet 21 CFR Part 11 standards: it lacks secure audit trails and advanced access control, leaving all data accessible and modifiable without oversight. To make up for these limitations, teams must create and maintain macros—a complex and time-consuming workaround. They tolerate it only for lack of a better alternative.
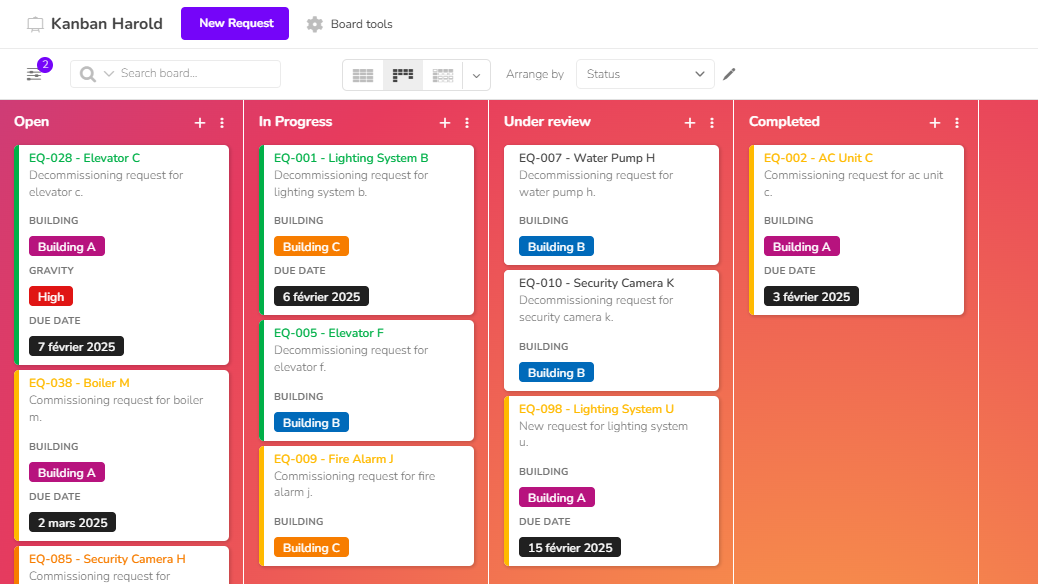



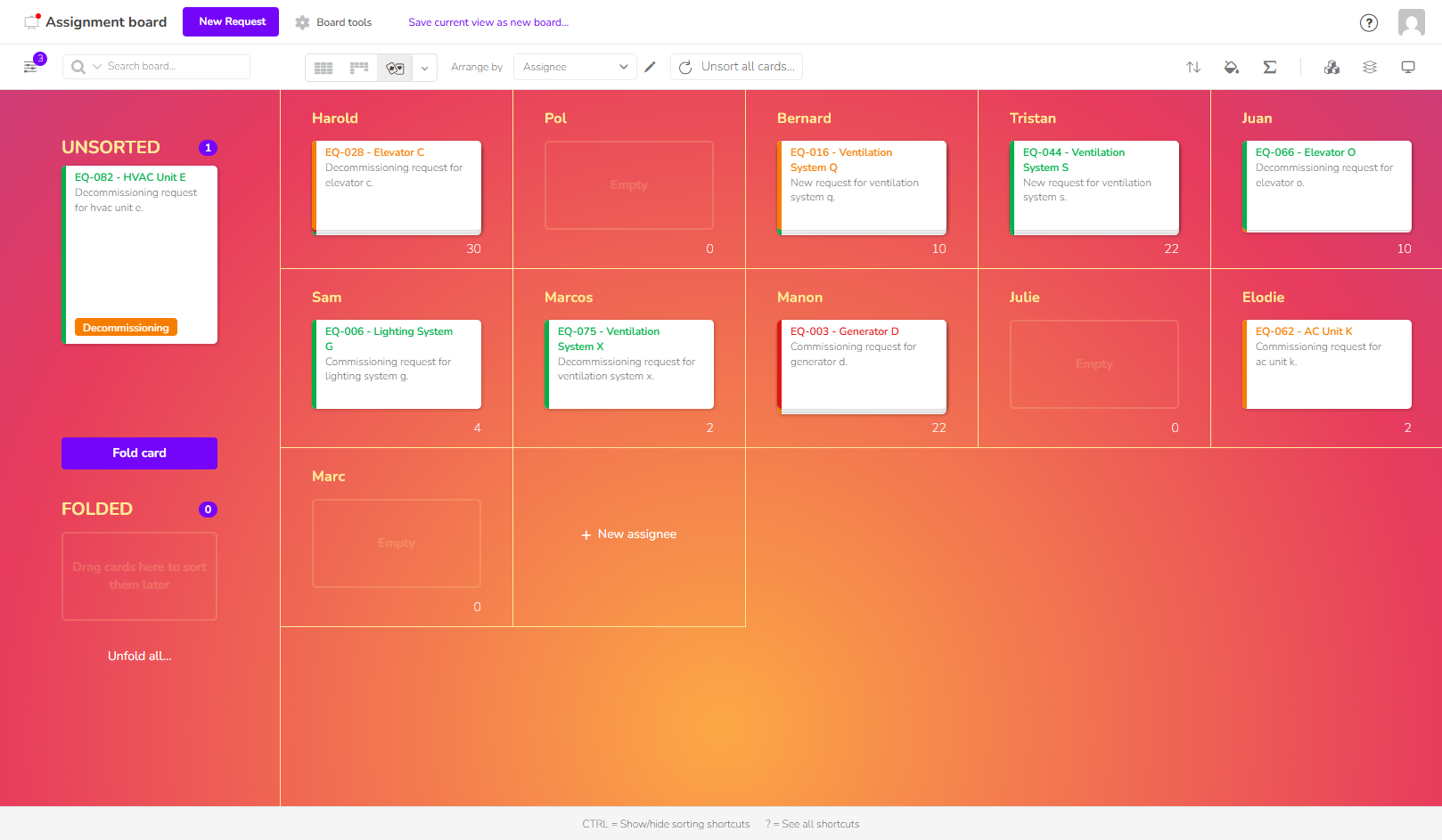
Avoid errors and information loss caused by scattered, unsynchronized tools. Klaro Cards brings all your data together in one place to ensure reliable traceability and improved compliance.
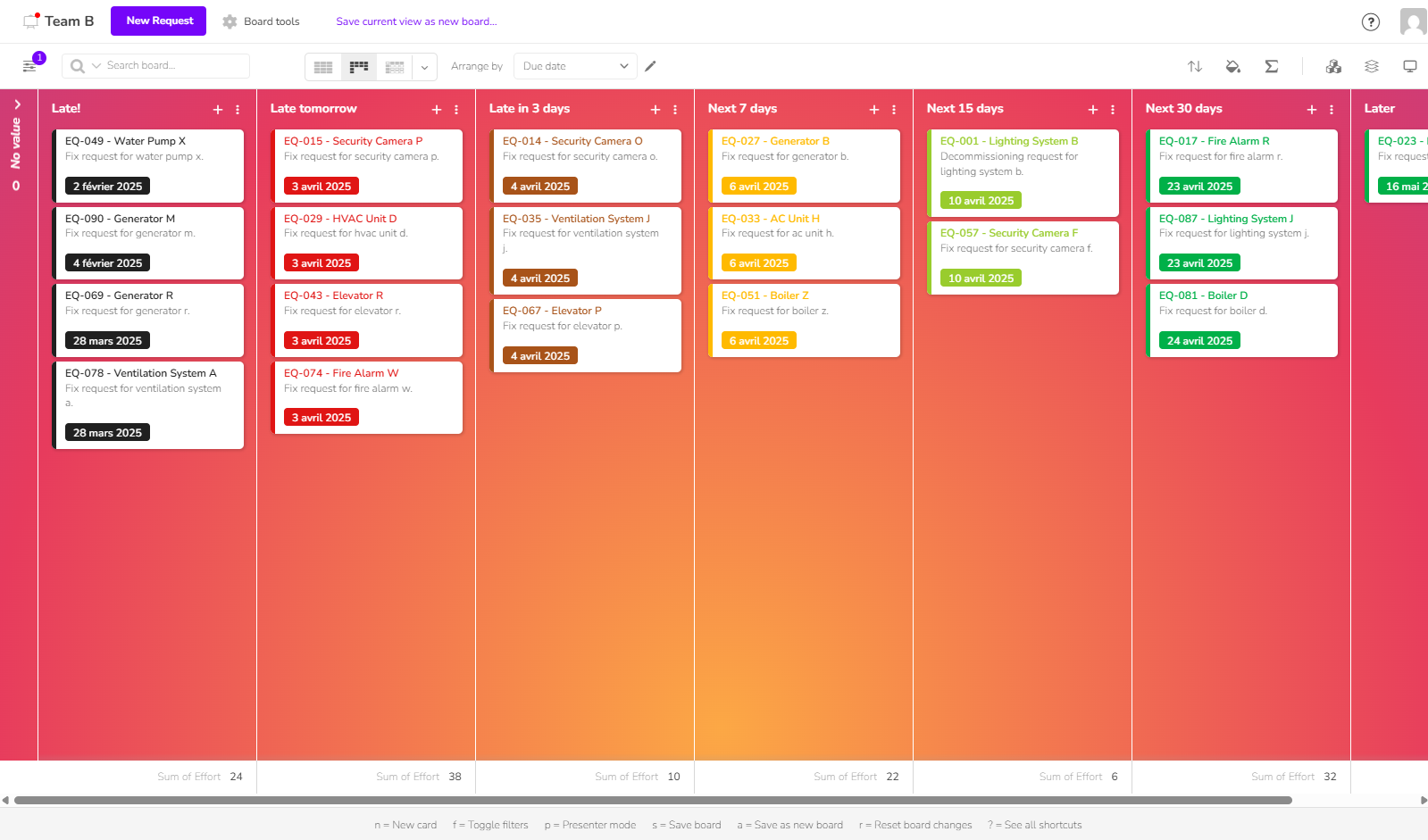
View, update, and share critical information about your validations, production batches, or clinical trials. Ideal for quality, R&D, and supply chain teams working across multiple sites or remotely, with a mobile version for field technicians.
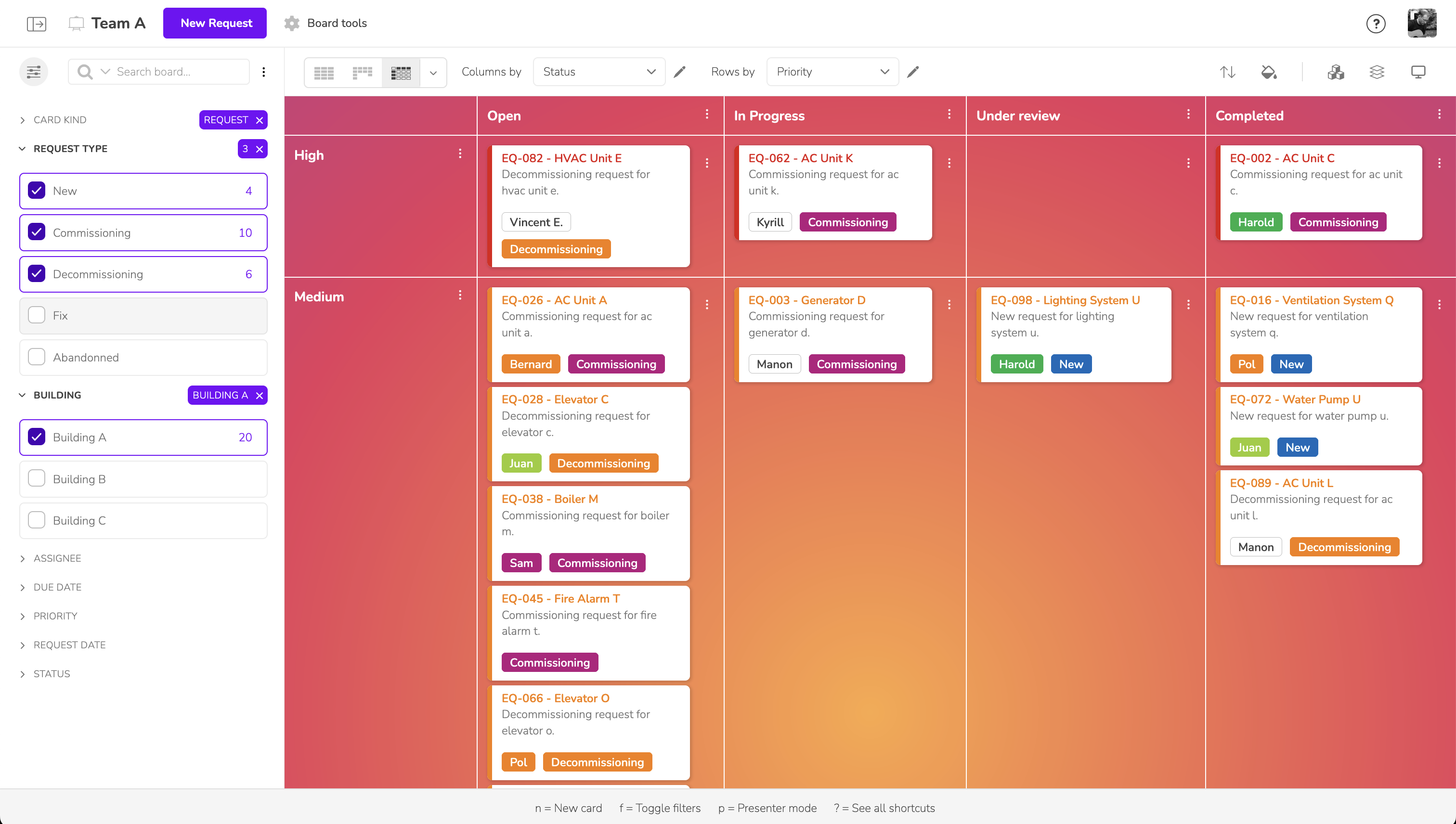
Display only the essential information based on your team, project phase, or current priorities. Klarify your Pharma lets you structure your data the way you need, giving each user a clear, tailored view.
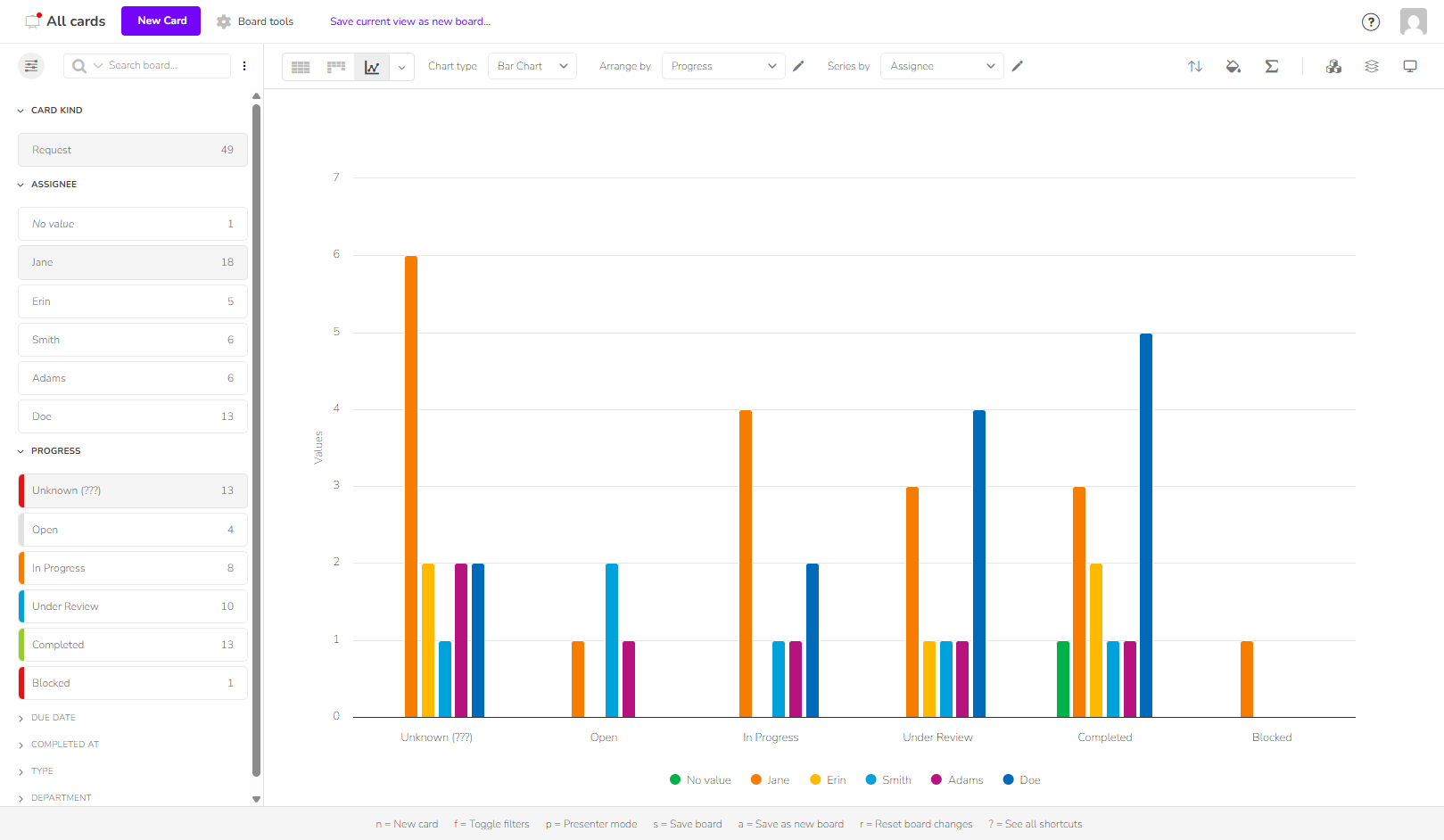
Give managers instant visibility into project progress without constantly relying on the teams. Klarify your Pharma centralizes key data and makes it accessible to everyone.
| Klarify your Pharma | Excel | |
|---|---|---|
| Regulatory compliance | Compliant with NIS2, 21 CFR Part 11, Eudralex Volume 4, Annex 11 | No action traceability, data easily modifiable |
| Real-time collaboration | Klarify your Pharma : instant updates, secure multi-user access | Risk of version conflicts, scattered files |
| Traceability and audit | Edit history, advanced access management | No audit trail, unsecured modifications |
| Simplicity and accessibility | Easy to use, no technical skills required | Requires complex macros to overcome limitations |
| Data volume management | Designed to handle and structure large amounts of information | Unstable with large files, risk of corruption |
| Configurable | Fully adaptable to business processes | Limited by formulas, rigid structure |
| Project and validation tracking | Centralized data, workflow automation | Manual processes, user-dependent |
Schedule a meeting to discuss your specific project management challenges.
Too much friction in communication? Lack of visibility on your priorities? Processes slowing execution? Share your challenges with us — we'll help you find clarity.
Our experts will show you live how Klarify your Pharma can streamline your organization and boost your productivity.